Facts about Ethanol

At gas stations, ethanol is contained in a mix of ethanol and gasoline, otherwise known as gasohol.

Pure or highly concentrated ethanol may permanently damage living tissue on contact.

Pure ethanol is a tasteless liquid with a strong and distinctive odor that produces a characteristic heat-like sensation when brought into contact with the tongue or mucous membranes.

Research shows that fuel consumption increases with the concentration of ethanol in a fuel blend.

Benzene, ethanol, and water form a ternary azeotrope with a boiling point of 64.9 °C.

During the fermentation process, it is important to prevent oxygen from getting to the ethanol, since otherwise the ethanol would be oxidised to acetic acid (vinegar).

The US uses Gasohol (max 10 percent ethanol) and E85 (85 percent ethanol) ethanol/gasoline mixtures.

Glucose for fermentation into ethanol can also be obtained from cellulose.

The bottoms from such a distillation is anhydrous ethanol, with several parts per million residual benzene.

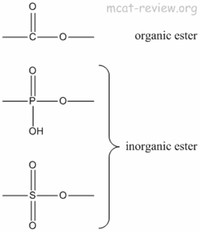
A very common ester of ethanol is ethyl acetate, found in for example nail polish remover.

The so obtained "neutralized ethanol" is then added to the target of the titration, which may be sample of neat organic acid.

Ethanol in the low concentrations typically found in most alcoholic beverages does not have useful disinfectant or antiseptic properties, internally or externally.
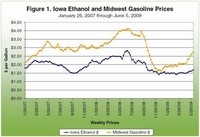
Later increases in petroleum prices, coupled with perennial uncertainty in agricultural prices, make forecasting the relative production costs of fermented versus petrochemical ethanol difficult.

Ethyl nitrite, prepared from the reaction of ethanol with sodium nitrite and sulfuric acid, was formerly a widely-used diuretic.

After distillation ethanol can be further purified by "drying" it using lime or salt.

Absolute ethanol and 95 percent ethanol themselves are good solvents, somewhat less polar than water and used in perfumes, paints and tinctures.

Some individuals have less effective forms of one or both of these enzymes, and can experience more severe symptoms from ethanol consumption than others.
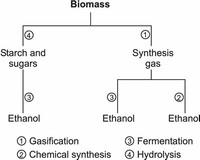
Use of these bacteria to produce ethanol from synthesis gas has progressed to the pilot plant stage at the BRI Energy facility in Fayetteville, Arkansas.

The ethanol molecule has a hydrophilic -OH group that helps it dissolve polar molecules and ionic substances.

Some researchers have warned that ethanol produced from agricultural feedstocks will cause a global food shortage, contributing to starvation in the Third World.

The mixture of 95.6 percent ethanol and 4.4 percent water (percentage by weight) is an azeotrope with a boiling point of 78.2 °C, and cannot be further purified by distillation.

Ethanol, also known as ethyl alcohol, drinking alcohol, or grain alcohol, is a flammable, colorless, slightly toxic chemical compound with a distinctive perfume-like odor.

The peak of the disinfecting power occurs around 70 percent ethanol; stronger and weaker solutions of ethanol have a lessened ability to disinfect.

The Canadian firm Iogen brought the first cellulose-based ethanol plant on-stream in 2004.

When heated to 150–220 °C over a silica- or alumina-supported nickel catalyst, ethanol and ammonia react to produce ethylamine.

Diethyl sulfate and triethyl phosphate, prepared by reacting ethanol with sulfuric and phosphoric acid, respectively, are both useful ethylating agents in organic synthesis.

Neutralized ethanol is used in order to compensate for this error.

Most salts of polyvalent ions are practically insoluble in ethanol.

An Australian study concluded that a 10 percent ethanol blend (E10) yielded a 2.6-2.8 percent increase in consumption.

At petroleum prices like those that prevailed through much of the 1990s, ethylene hydration was a decidedly more economical process than fermentation for producing purified ethanol.

Ethyl halides can also be produced by reacting ethanol by more specialized halogenating agents, such as thionyl chloride for preparing ethyl chloride, or phosphorus tribromide for preparing ethyl bromide.

S. cynthia is known as the ailanthus silkmoth, since it feeds on the leaves of the Ailanthus genus.

Ethanol applied to unbroken skin cools the skin rapidly through evaporation.

Millions more acres of land are needed if ethanol is to be used to replace gasoline.

The ethanol-water azeotrope can be broken by the addition of a small quantity of benzene.

Absolute ethanol produced this way has no residual benzene, and can be used to fortify port and sherry in traditional winery operations.

The ethanol content of a beverage is usually measured in terms of the volume fraction of ethanol in the beverage, expressed either as a percentage or in alcoholic proof units.

Other proportions of ethanol with water or other solvents can also be used as a solvent.

Applejack is traditionally made by freeze distillation: water is frozen out of fermented apple cider, leaving a more ethanol-rich liquid behind.

The amount of ethanol in the body is typically quantified by blood alcohol content (BAC), the milligrams of ethanol per 100 milliliters of blood.

Ethanol-water mixtures have less volume than their individual components: a mixture of equal volumes ethanol and water has only 95.6 percent of the volume of equal parts ethanol and water, unmixed.
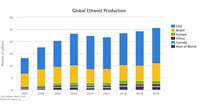
World production of ethanol in 2015 was 24.7 billion gallons, with 88 percent of the world supply coming from Brazil and the United States.

Ethanol has been shown to increase the growth of Acinetobacter baumannii, a bacterium responsible for pneumonia, meningitis and urinary tract infections.

Absolute or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent water.

Pure ethanol is classed as 200 proof in the United States, equivalent to 175 degrees proof in the (now rarely used) UK system.

Ethanol produced from corn has a number of critics who suggest that it is primarily just recycled fossil fuels because of the energy required to grow the grain and convert it into ethanol.

Vinegar is a dilute solution of acetic acid prepared by the action of Acetobacter bacteria on ethanol solutions.

The two largest-volume ethyl esters are ethyl acrylate (from ethanol and acrylic acid) and ethyl acetate (from ethanol and acetic acid).

Ethanol is produced both as a petrochemical, through the hydration of ethylene, and biologically, by fermenting sugars with yeast.

Ethanol has been used as fuel in bipropellant rocket vehicles, in conjunction with an oxidizer.

Infrared ethanol sensors measure the vibrational frequency of dissolved ethanol using the CH band at 2900cm-1.

Conversely, those who have acquired ethanol tolerance have a greater quantity of these enzymes, and metabolize ethanol more rapidly.

Fractional distillation can concentrate ethanol to 95.6 percent by weight (89.5 mole percent).

At the molecular level, liquid ethanol consists of hydrogen-bonded pairs of ethanol molecules; this phenomenon renders ethanol more viscous and less volatile than less polar organic compounds of similar molecular weight.

When ethanol is produced as a mixing beverage it is a neutral grain spirit.

In breweries and biofuel plants, the quantity of ethanol present is measured using one of two methods.

Small doses of ethanol generally produce euphoria and relaxation; people experiencing these symptoms tend to become talkative and less inhibited, and may exhibit poor judgment.

Ethanol in the past has been used commercially to synthesize dozens of other high-volume chemical commodities.

Ethanol is easily soluble in water in all proportions with a slight overall decrease in volume when the two are mixed.

Ethanol can be quantitatively converted to its conjugate base, the ethoxide ion (CH3CH2O?), by reaction with an alkali metal such as sodium.
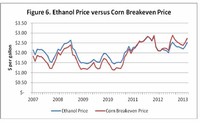
Apart from this, the amount of ethanol needed to run the United States, for example, is greater than its own farmland could produce, even if fields used for food were converted into cornfields.

Ethanol, like most short-chain alcohols, is flammable, colorless, has a strong odor, and is volatile.

Ethanol is also used in design and sketch art markers, such as Copic, and Tria.

Cornmeal and other plant-derived absorbents cannot readily be regenerated, but where ethanol is made from grain, they are often available at low cost.

When lime (calcium oxide) is mixed with the water in ethanol, calcium hydroxide forms.

The product of either ethylene hydration or brewing is an ethanol-water mixture.

Other yeasts and bacteria are under investigation to metabolize xylose and so improve the ethanol yield from cellulosic material.

Currently the main feedstock in the United States for the production of ethanol is corn.

Advocates for wheat, corn, and sugar growers have succeeded in their attempts to lobby for regulatory intervention encouraging adoption of ethanol, stimulating debate over who the major beneficiaries of increased use of ethanol would be.

Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62 percent (percentage by weight, not volume) as an antiseptic.
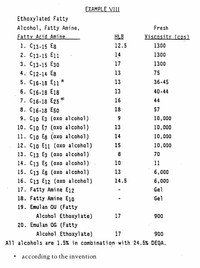
Organic solids of low molecular weight are usually soluble in ethanol.

The membrane can break the water-ethanol azeotrope because separation is not based on vapor-liquid equilibria.
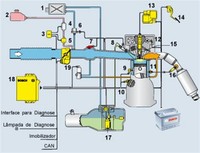
Flex fuel engines are able to work with all ethanol, all gasoline or any mixture of both, giving the buyer a choice for a perfect balance between price/performance issue.

The catalyst is most commonly phosphoric acid, adsorbed onto a porous support such as diatomaceous earth or charcoal; this catalyst was first used for large-scale ethanol production by the Shell Oil Company in 1947.

Vinegar made from distilled ethanol is called "distilled vinegar," and is commonly used in food pickling and as a condiment.

Fermented beverages may contain up to 15–25 percent ethanol by volume, the upper limit being set by the yeast's tolerance for ethanol, or by the amount of sugar in the starting material.

Today almost 50 percent of Brazilian cars are able to use 100 percent ethanol as fuel, that includes ethanol only engines and flex fuel engines.

Ethanol can be oxidized to acetaldehyde, without overoxidation to acetic acid, by reacting it with pyridinium chromic chloride.

Some medications, including paracetamol (acetaminophen), as well as exposure to organochlorides, can deplete the body's glutathione supply, enhancing both the acute and long-term risks of even moderate ethanol consumption.

Ethanol can be oxidized to acetaldehyde, and further oxidized to acetic acid.

The most ethanol-tolerant strains of yeast can survive in up to about 15 percent ethanol (by volume).

Approximately 2.8 gallons of ethanol (10 liters) are produced from one bushel of corn (35 liters).

Ethanol's hydroxyl proton is weakly acidic, having a pKa of only 15.9, compared to water's 15.7 (Ka of ethanol is a measure of .

Pure ethanol has a lower energy content than gasoline (about 30 percent less energy per unit volume).

The ethanol molecule has a hydrophilic -OH group that helps it dissolve polar molecules and ionic substances.

The initial product of ethanol metabolism, acetaldehyde, is more toxic than ethanol itself.

Others, however, disagree, pointing out that ethanol production does not necessarily have to come from the farming of corn.
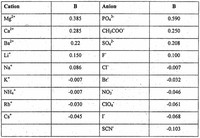
Among ionic compounds, many monovalent salts are at least somewhat soluble in ethanol, with salts of large, polarizable ions being more soluble than salts of smaller ions.

At higher dosages (BAC > 100mg/dl), ethanol acts as a central nervous system depressant, producing at (progressively higher dosages) impaired sensory and motor function, slowed cognition, stupefaction, unconsciousness, and possible death.

Alcoholic drinks sometimes feature flavor of mint, namely the Mint Julep and the Mojito.

Once the non-polar material is dissolved in the ethanol, water can be added to prepare a solution that is mostly water.

The indicator (phenolphthalein, for example) is added to the ethanol solvent first and KOH is added until the color of the solution turns pale pink.

The addition of even a small amount of ethanol to water sharply reduces the surface tension of water.

Dry salt will dissolve some of the water content of the ethanol as it passes through, leaving a purer alcohol.

Brewing can only produce relatively dilute concentrations of ethanol in water; concentrated ethanol solutions are toxic to yeast.

The largest national fuel ethanol industries exist in Brazil (gasoline sold in Brazil contains at least 20 percent ethanol and hydrous ethanol is also used as fuel).