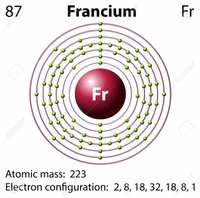
Facts about Francium
The francium nuclei typically last for three minutes and must be trapped and observed before they decay.

The second naturally occurring isotope of francium is 224Fr, a member of the thorium radioactive series.

All known isotopes of francium are highly unstable, therefore knowledge of the properties of this element comes only from radiochemical procedures.

A small number of images of francium have been obtained, but of at most 350,000 atoms at a time.

Many radioactive isotopes of francium have been produced, with atomic mass numbers between 199 and 232.

Francium is the least electronegative of all the known elements, with cesium being the second least.

The nuclei of 18O were accelerated to an energy of 100 million electron volts (MeV), to give them sufficient energy to fuse with gold nuclei and produce francium nuclei.

Francium is formed and occurs as a result of the radioactive decay (alpha decay) of actinium.

Francium lies in group 1 (former group 1A)—the alkali metal group—of the periodic table.