Facts about Lithium

Commercial production of lithium metal was achieved in 1923 by a German company (Metallgesellschaft), by the electrolysis of molten lithium chloride and potassium chloride.

Today, most commercial lithium is recovered from brine sources in Argentina and Chile.

In 1818, Christian Gmelin was the first to observe that lithium salts give a bright red color when held in a flame.

Lithium has only about half the density of water, because of which sticks of this metal have the odd heft of a light wood such as balsa.

Some jurisdictions limit the sale of lithium batteries, which are the most readily available source of lithium metal for regular consumers.

The Earth's crust contains about 65 parts per million (ppm) of lithium.

Continued expansion in the portable electronic products market and commercialization of hybrid electric vehicles using lithium batteries suggest growth of up to 10 percent per year in lithium carbonate consumption in this market through 2010.

The name "Malaysia" was adopted in 1963 when the Federation of Malaya, Singapore, Sabah, and Sarawak formed a 14-state federation.

Most consumer lithium batteries, however, have built-in thermal overload protection to prevent this type of incident, or their design limits short-circuit currents.

Like all other alkali metals, lithium has a single electron in its outermost shell, and it can readily lose this electron to become a positive ion.

The metal is produced by electrolysis from a mixture of fused (molten) lithium chloride and potassium chloride.

The element was not isolated until William Thomas Brande and Sir Humphry Davy later performed electrolysis on lithium oxide in 1818.

Lithium niobate is used in mobile phones, lithium stearate is a high-temperature lubricant, lithium hydroxide is an efficient air purifier, and lithium chloride and bromide are used as desiccants.

The shortest-lived isotope of lithium is 4Li, which decays through proton emission and has a half-life of 7.58043x10-23 seconds.

Lithium can be used to reduce pseudoephedrine and ephedrine to methamphetamine by the Birch reduction method, which employs alkali metals dissolved in ammonia.

Lithium fires are difficult to extinguish, requiring special chemicals designed to smother them.

Between 2002 and 2005, lithium minerals production rose by 7 percent per year to reach 18,800 tons lithium.

Consumption of lithium increased by 4-5 percent per year between 2002 and 2005, driven by the demand in lithium secondary batteries.

China may emerge as a significant producer of brine-based lithium carbonate by 2010.

When Johan Arfvedson analyzed a petalite ore in 1817, he discovered lithium.
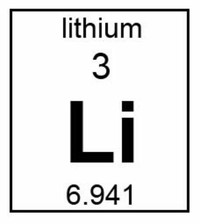
Lithium (chemical symbol Li, atomic number 3) is the lightest solid chemical element and a member of the group of elements known as alkali metals.

Lithium is soft enough to be cut with a knife, though this is significantly more difficult to do than cutting sodium.

When placed over a flame, lithium gives off a striking crimson color, but when it burns strongly, the flame becomes brilliant white.

The atomic number of lithium is 3, which places it right after helium (atomic number 2).

Given that the specific heat capacity of lithium is higher than that of any other solid, lithium is used in heat-transfer applications, such as in toasters and microwave ovens.
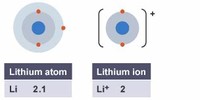
By contrast, lithium (in the ionic form) appears to be an essential trace element for goats and possibly rats.

Naturally occurring lithium is composed of 2 stable isotopes, 6Li and 7Li, of which the latter is the more abundant (92.5% natural abundance).

Carriage and shipment of some types of lithium batteries may be prohibited aboard aircraft, because most types of lithium batteries can discharge very rapidly when short-circuited, leading to overheating and possible explosion.

Lithium leads the family of elements known as "alkali metals" in group 1 of the periodic table.

Toward the end of the 1700s, Brazilian scientist Josй Bonifбcio de Andrada e Silva discovered the lithium-containing mineral petalite (LiAl(Si2O5)2) on a trip to Sweden.

Robert Fitzgerald has striven to situate the Iliad in the musical forms of English poetry.
Lithium, atomic number 3, is an element of many uses. It's used in aircraft manufacture and in certain batteries. It's also used in mental health: Lithium carbonate is a common treatment of bipolar disorder, helping to stabilize the wild mood swings caused by the illness.Sep 23, 2015
Lithium affects the flow of sodium through nerve and muscle cells in the body. Sodium affects excitation or mania. Lithium is used to treat the manic episodes of bipolar disorder. Symptoms include hyperactivity, rushed speech, poor judgment, reduced need for sleep, aggression, and anger.
Lithium is the first member of the alkali metal family. ... Lithium has a number of important and interesting uses. In recent years, it has been used to make lightweight, efficient batteries. Compounds of lithium have also been used to treat a mental disorder known as bipolar disorder.
While being the lightest metal under normal conditions, it is still the most dense. With an atomic number of three, lithium has three protons in the nucleus, but like many other alkalai metals it has only one valence electron. Interesting Lithium Facts: It is the least reactive of the alkalai metals.
Instead, lithium is usually extracted from lithium minerals that can be found in igneous rocks (chiefly spodumene) and from lithium chloride salts that can be found in brine pools. [4] The largest producer of lithium in the world is Chile, which extracts it from brine at the Atacama Salt Flat.Nov 30, 2010
Lithium does not occur as the metal in nature, but is found combined in small amounts in nearly all igneous rocks and in the waters of many mineral springs. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium.
Lithium can cause nausea, diarrhea, dizziness, muscle weakness, fatigue, and a dazed feeling. These unwanted side effects often improve with continued use. Fine tremor, frequent urination, and thirst can occur and may persist with continued use. Weight gain and swelling from excess fluid can also occur.
Lithium - Dietary. Lithium is a highly reactive, light metal naturally found in very low levels throughout the body. It is available as a dietary supplement and is commonly found in drinking water and in many foods, including grains, vegetables, mustard, kelp, pistachios, dairy, fish, and meat.Jul 26, 2016
► Keep your caffeine intake about the same. Keep amounts of coffee, tea, cola, and other soft drinks with caffeine about the same from day to day. Less caffeine can cause your lithium level to increase; more caffeine can cause your lithium level to decrease. ► Avoid alcoholic beverages.





