Facts about Matter

The Standard Model's definition of matter proves to be incomplete in accounting for mass at the most fundamental levels of matter.

In bulk, matter can exist in several different phases, according to the conditions of pressure and temperature.

Entities not considered to be matter include the virtual particles carrying the fundamental forces of nature; light (photons), which are the carrier of the electromagnetic force, and other gauge bosons.

Additional phases include plasmas, superfluids, supersolids, Bose-Einstein condensates, fermionic condensates, liquid crystals, strange matter, and quark-gluon plasmas.

The various types of energy and force fields are not usually considered matter per se, although force fields may contribute to the mass of objects.

A possible definition of matter that at least some physicists use is that it is everything that is constituted of elementary fermions.
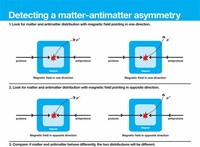
The apparent asymmetry of matter and antimatter in the visible universe is one of the great unsolved problems in physics.

Antimatter comprises collections of antiparticles existing in forms corresponding to the forms of normal matter.

Given that protons, neutrons, and electrons combine to form atoms, one can say that atoms, molecules, and the bulk substances that they make up are all part of "matter."

Phases are sometimes called states of matter, but this term can lead to confusion with thermodynamic states.

Matter is commonly thought of as the material that composes physical objects—that is, objects that have mass and occupy space.

Antimatter is not found naturally on Earth, except very briefly and in vanishingly small quantities (as the result of radioactive decay or cosmic rays).

Antiparticles and some stable antimatter (such as antihydrogen) can be made in tiny amounts, but not in enough quantity to do more than test a few of its theoretical properties.
Snow is water that crystallizes when the temperature gets below freezing. Sleet is when the temperature freezes, but then as it falls from the clouds it partially melts. Clouds actually contain 2 states of matter, solid and gas. Rain is liquid.
Matter is a substance that has inertia and occupies physical space. According to modern physics, matter consists of various types of particles, each with mass and size. The most familiar examples of material particles are the electron, the proton and the neutron. Combinations of these particles form atoms.
Matter is made of tiny little particles, too. The particles that make up matter are called atoms. You cannot see atoms because they are so small. Lots of atoms join together to make up matter that you can see.
Solids, liquids and gases are called the three states of matter. Materials can be changed from one state to another by heating or cooling. Water can be observed as a liquid, a solid (ice), or a gas (water vapour) and moves around the environment in a process known as the water cycle.
Physical properties of matter can be observed and tested. They include properties such as color, length, volume, odor, and density. These properties are extensive if they depend on the amount of the substance being used or intensive if they do not depend on the amount of substance being used.
1.The three basic properties of matter are volume, mass, and shape. ... All matter is made up of tiny particles called atoms. ... Volume is the amount of space that matter takes up. ... Mass is the amount of matter an object has. ... Liquids take the shape of their container.
PLAYproperties of matter. solid, liquid, gas.atoms. tiny particles that make up matter.volume. amount of space that matter takes up.mass. the amount of matter an object has.liquids. take the shape of their container.gases. do not have a definite shape or volume.liquids. ... solids.More items...
Some examples of physical properties are:color (intensive)density (intensive)volume (extensive)mass (extensive)boiling point (intensive): the temperature at which a substance boils.melting point (intensive): the temperature at which a substance melts.
There are four different properties of matter. They are weight, volume, mass, and density. The most important one is mass. Mass is the amount of matter in an object and it never changes unless matter is taken out of the object.
Intensive properties: A physical property that will be the same regardless of the amount of matter.density:color: The pigment or shade.conductivity: electricity to flow through the substance.malleability: if a substance can be flattened.luster: how shiny the substance looks.
Extensive properties depend on the amount of matter that is being measured. These include mass and volume. Intensive properties do not depend on the amount of matter. These include density and color.
