Facts about Molybdenum

The Russian Luna 24 mission discovered a single grain (1 Ч 0.6 micrometer) of pure molybdenum in a pyroxene fragment taken from Mare Crisium on the Moon.

Until the late eighteenth century, the compounds of molybdenum were confused with those of other elements, such as carbon or lead.

The element molybdenum (from the Greek molybdos, meaning "lead-like") is not found free in nature.

Molybdenum in trace amounts has been found to have a role in the biology of all classes of organisms.

Plants and animals generally have molybdenum present in amounts of a few parts per million.

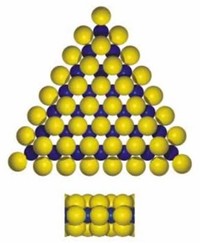
Molybdenum (chemical symbol Mo, atomic number 42) is a silvery white, soft metal.

Molybdenum has six stable isotopes and many radioisotopes, most of which have very short half-lives.

Molybdenum is obtained by mining molybdenite directly and is also recovered as a byproduct of copper mining.

Molybdenum has been found to have a role in the biology of all classes of organisms.

Molybdenum use soared during World War I, when the increased demand for tungsten made that element scarce and high-strength steels were at a premium.
The molybdenum atom is present in a cluster that includes iron and sulfur atoms.

Molybdenum was little used and remained in the laboratory until the late nineteenth century.
Molybdenum dusts and some molybdenum compounds, such as molybdenum trioxide and water-soluble molybdates, may have slight toxicities if inhaled or ingested orally.

Regulations by the U.S. Occupational Safety and Health Administration (OSHA) specify maximum molybdenum exposure in an eight-hour day (40-hour week) to be 15 milligrams (mg) per cubic meter.

In 1778, Carl Wilhelm Scheele was able to determine that molybdenum was separate from graphite and lead, and he isolated the oxide of the metal from molybdenite.

In animals, molybdenum is a cofactor of the enzyme xanthine oxidase, which is involved in certain metabolic pathways (purine degradation and formation of uric acid).

About half of the world's molybdenum is mined in the United States.

Subsequently, a French company (Schneider and Co.) tried molybdenum as an alloying agent in steel armor plating and noted its usefulness as a hardener of steel.

Laboratory tests suggest, however, that molybdenum is of relatively low toxicity, compared to many heavy metals.

Pure molybdenum has a melting point of 2623°C, which is among the highest melting points of all elements.

Molybdenum disulfide is a good lubricant, and molybdenum pigments are used in paints, inks, plastics, and rubber compounds.

The molybdenum causes excretion of copper reserves from the animal, leading to copper deficiency.

In 1778, Carl Wilhelm Scheele was able to determine that molybdenum was separate from graphite and lead, and he isolated the oxide of the metal from molybdenite.

Molybdenum is a transition metal that lies in period five of the periodic table, between niobium and technetium.

In young calves, the molybdenum toxicity is manifested as "teart" or shooting diarrhea, where the dung is watery, full of air bubbles and with a fetid odor.
