Facts about Ruthenium

A hard, white metal, ruthenium does not tarnish at normal temperatures, but under certain conditions it oxidizes explosively.

Small amounts of ruthenium can increase the hardness of platinum and palladium.

Seven stable isotopes of ruthenium have been found in nature: 96Ru, 98Ru, 99Ru, 100Ru, 101Ru, 102Ru, and 104Ru.

Ruthenium produced in such a way contains radioactive isotopes, some with a half-life of up to 373.59 days.
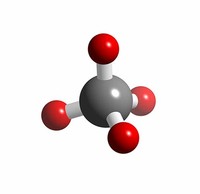
The compound ruthenium tetroxide (RuO4), similar to osmium tetroxide, is highly toxic and may explode.

The ruthenium organometallic compound easiest to make is RuHCl(CO)(PPh3)3.

Ruthenium (chemical symbol Ru, atomic number 44) is a rare, hard, white metal.

Ruthenium readily forms organometallic compounds in which its atoms are directly bonded to carbon atoms.
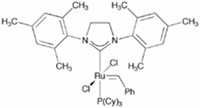
Grubbs' catalyst and Roper's complex are two of the important organometallic catalysts based on ruthenium.

