Facts about Sodium

Common salt, or table salt, is sodium chloride, widely used as a food flavoring.

From the weight of the sodium salt and the volume of air in the room, we easily calculate that one part by weight of air could not contain more than 1/20 millionth weight of sodium."

Sodium hydroxide (NaOH), also known as lye or caustic soda, is a strong chemical base, widely used in chemical laboratories and in industry.

An alloy of sodium and potassium (NaK) is a heat-transfer medium and a chemical reducing agent.

When sodium or its compounds are burned in a flame, they give off a distinct yellow color, corresponding to two main spectral lines—the "sodium D lines" at wavelengths 588.9950 and 589.5924 nanometers.

Sodium hydroxide does not attack iron or copper, but many other metals—such as aluminum, zinc, and titanium—are attacked rapidly.

Laboratory flasks and glass-lined chemical reactors are damaged by long exposure to hot sodium hydroxide, and the glass becomes frosted.

Unfortunately, many people ingest sodium chloride (table salt) in excess, which can have negative effects on health.

Sodium hydroxide slowly reacts with glass to form sodium silicate, so glass joints and stopcocks exposed to NaOH have a tendency to "freeze."

After a few minutes, the flame gradually turned yellow and showed a strong sodium line that disappeared only after 10 minutes.

At a pressure of 30 gigapascals (300,000 times sea-level atmospheric pressure), the melting temperature of sodium begins to drop.

Under extreme pressure, sodium departs from standard rules for changing to a liquid state.
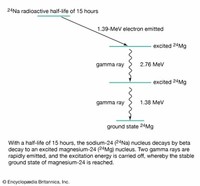
Sodium has two radioactive cosmogenic isotopes—that is, isotopes produced when high-energy cosmic rays interact with the nuclei of sodium atoms.

Sodium is the most abundant alkali metal and makes up about 2.6 percent by weight of the Earth's crust.

Like the other alkali metals, sodium metal is a soft, lightweight, reactive metal.

When exposed to an acid, sodium bicarbonate releases carbon dioxide and water.

Sodium carbonate (Na2CO3), also known as washing soda or soda ash, is a sodium salt of carbonic acid (H2CO3).

The metal itself was first isolated in 1807, when Sir Humphry Davy carried out the electrolysis of caustic soda (sodium hydroxide).

Related to this is the reaction of sodium hydroxide with acidic oxides such as carbon dioxide (CO2) and sulfur dioxide (SO2).

The carbonate, chloride, and hydroxide of sodium are important industrial chemicals, used in the manufacture of glass, pulp and paper, textiles, soaps and detergents, and other sodium salts.

Gloves and eye protection should be worn when using sodium hydroxide, because it can cause chemical burns, injury or scarring, and blindness.

The concentration of sodium ions in the blood is directly related to the regulation of safe body-fluid levels.

Caustic soda is produced by the electrolysis of an aqueous solution of sodium chloride.

The symbol for sodium, Na, comes from the neo-Latin name for a common sodium compound called natrium.

Sodium bicarbonate (NaHCO3) is also known as sodium hydrogen carbonate, or bicarbonate of soda, or baking soda.

Sodium metal floats on water and reacts violently with it, releasing heat, hydrogen gas, and caustic sodium hydroxide solution.

At the end of the nineteenth century, sodium was chemically prepared by heating sodium carbonate with carbon to 1,100 °C.

Sodium metal can be used to isolate some other metals from their compounds, smoothen metal surfaces, and manufacture several compounds.

The hydroxide ion makes sodium hydroxide a strong base, which reacts with acids to form water and the corresponding salts.

Sodium bicarbonate is an antacid, a leavening agent in baking, and an ingredient in some forms of toothpaste.

The propagation of nerve impulses (by "signal transduction") is regulated by sodium ions.

Sodium chloride, also known as common salt or table salt, is a compound with the chemical formula NaCl.

Sodium (chemical symbol Na, atomic number 11) is a member of a group of chemical elements known as alkali metals.

Given its reactivity, sodium is not found as a free metal in nature but is bound to other elements in the form of compounds.

The most common sodium-containing mineral is halite (or rock salt), chemically known as sodium chloride.

Sodium hydroxide is a powerful chemical base, but sodium bicarbonate and sodium carbonate are also used to raise the alkalinity of solutions.

Sodium hydroxide is an ionic compound, made up of sodium ions and hydroxide ions.

Sodium is now produced commercially through the electrolysis of fused (liquefied) sodium chloride.
Sodium is dissolved in the blood and plays a key role in maintaining blood pressure. Sodium attracts and holds water, so the sodium in the blood helps maintain the liquid portion of the blood. On the other hand, if you consume too much sodium, your body may hold onto extra water, increasing the volume of your blood.
Sodium is an essential electrolyte that helps maintain the balance of water in and around your cells. It's important for proper muscle and nerve function. It also helps maintain stable blood pressure levels. Insufficient sodium in your blood is also known as hyponatremia.Mar 28, 2017
Sodium vapor is used in streetlights and produces a brilliant yellow light. Sodium also forms many useful compounds. Some of the most common are: table salt (NaCl), soda ash (Na2CO3), baking soda (NaHCO3), caustic soda (NaOH), Chile saltpeter (NaNO3) and borax (Na2B4O. 10H2O).
It is the sixth most abundant metal in the Earth's crust. Sodium was first isolated from sodium hydroxide by Humphry Davy in 1807. Sodium is a vital element for living organisms. Of the twenty known isotopes of sodium, only one—Na-23—is stable.
Sodium is used as a heat exchanger in some nuclear reactors, and as a reagent in the chemicals industry. But sodium salts have more uses than the metal itself. The most common compound of sodium is sodium chloride (common salt). It is added to food and used to de-ice roads in winter.
Sodium was first isolated in 1807 by Sir Humphry Davy, who made it by the electrolysis of very dry molten sodium hydroxide, NaOH. Sodium collected at the cathode. Davy isolated potassium by a similar procedure, also in 1807.
High-Sodium Foods:Smoked, cured, salted or canned meat, fish or poultry including bacon, cold cuts, ham, frankfurters, sausage, sardines, caviar and anchovies.Frozen breaded meats and dinners, such as burritos and pizza.Canned entrees, such as ravioli, spam and chili.Salted nuts.Beans canned with salt added.
Having less sodium in your diet may help you lower or avoid high blood pressure. People with high blood pressure are more likely to develop heart disease or have a stroke. Most people eat too much sodium, often without knowing it. The average American eats about 3,400 mg of sodium a day.
With weight loss advice being so focused on eliminating bad sugar from our diet, it's time to realize that salt is just as bad. ... Studies have shown that an increase in sodium levels has a direct influence on blood pressure, which is why having a diet lower in sodium is critical to maintain healthy weight loss.







