Facts about Sulfur

Given its flammable nature, sulfur also finds use in matches, gunpowder, and fireworks.

In 1867, sulfur was discovered in underground deposits in Louisiana and Texas.

Early alchemists gave sulfur its own alchemical symbol—a triangle at the top of a cross.

The burning of coal and petroleum by industry and power plants liberates huge amounts of sulfur dioxide (SO2), which reacts with atmospheric water and oxygen to produce sulfuric acid.

Many of the unpleasant odors of organic matter, including garlic odor and "skunk stink," are produced by sulfur-containing compounds.

Sulfur (Sanskrit, sulvere; Latin sulpur) was known in ancient times, and is referred to in several books of the Bible, including the book of Genesis.

Sulfur is also used in batteries, detergents, the vulcanization of rubber, fungicides, and the manufacture of phosphate fertilizers.

The International Union of Pure and Applied Chemists (IUPAC) adopted the spelling "sulfur" in 1990, as did the Royal Society of Chemistry Nomenclature Committee in 1992.

Inorganic sulfur forms a part of iron-sulfur clusters, and sulfur is the bridging ligand in the CuA site of the enzyme cytochrome c oxidase.

Sulfur or sulphur (see spelling below) (chemical symbol S, atomic number 16) is a yellow crystalline solid at ordinary temperatures and pressures.

Sulfur extracted from oil, gas, and the Athabasca Oil Sands has led to a glut on the market, and huge stockpiles of sulfur can be seen throughout Alberta.

Sulfur is used as the light-generating medium in the rare lighting fixtures known as sulfur lamps.

Sulfur is insoluble in water but soluble in carbon disulfide and, to a lesser extent, in other organic solvents such as benzene.

Sometime in the twelfth century, the Chinese invented gunpowder, which is a mixture of potassium nitrate (KNO3), carbon, and sulfur.

The amino acids homocysteine and taurine also contain sulfur, but they are not part of the primary structure of proteins.

Carbon disulfide, carbon oxysulfide, hydrogen sulfide, and sulfur dioxide should all be handled with care.

Sulfur can combine with other elements in different proportions, and it is therefore described as being multivalent.

Amorphous or "plastic" sulfur can be produced through the rapid cooling of molten sulfur.

Sulfur dioxide is sufficiently safe to be used as a food additive in small amounts, but at high concentrations it reacts with moisture to form sulfurous acid.
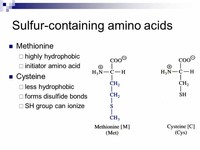
The amino acids cysteine and methionine contain sulfur, as do all peptides and proteins that contain these amino acids.

Sulfur has 18 isotopes, of which four are stable: 32S (95.02 percent), 33S (0.75 percent), 34S (4.21 percent), and 36S (0.02 percent).

Fuel standards increasingly require sulfur to be extracted from fossil fuels, to prevent the formation of acid rain.

Elemental sulfur can be found near hot springs and volcanic regions in many parts of the world, especially along the Pacific "Ring of Fire"—a zone of frequent earthquakes and volcanic eruptions encircling the Pacific Ocean.

Furniture makers of the late eighteenth century used molten sulfur to produce decorative inlays in their craft.

The consumption of sulfuric acid has been regarded as one of the best indices of a nation's industrial development.

That craft, however, was soon abandoned because of the sulfur dioxide produced during the process of melting sulfur.

The element has traditionally been spelled sulphur in several countries, such as the United Kingdom, Ireland, Hong Kong, and India, but it is spelled sulfur in the United States.

When it burns, sulfur produces a blue flame and emits sulfur dioxide—a gas that is notable for its peculiar, suffocating odor, like that of burnt matches.

Removing one atom from the crown gives S7, which is responsible for sulfur's distinctive yellow color.

More sulfuric acid is produced in the United States every year than any other industrial chemical.

Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydrologic studies.

The viscosity of molten sulfur, unlike that of most other liquids, increases with temperature because of the formation of polymer chains.

Significant deposits of elemental sulfur also exist in salt domes along the coast of the Gulf of Mexico and in evaporites in Eastern Europe and western Asia.

Sulfur is absorbed by plants via the roots from soil as the sulfate ion and reduced to sulfide before being incorporated into cysteine and other organic sulfur compounds—a process called sulfur assimilation.

Selenium, the heavier analog of sulfur, can form rings but is more often found as a polymer chain.
Sulfur is the third most abundant mineral in the body, about half being concentrated in the muscles, skin and bones, and is essential for life. Sulfur makes up vital amino acids used to create protein for cells, tissues, hormones, enzymes, and antibodies.
Sulfur is also used in the vulcanization of natural rubber, as a fungicide, in black gunpowder, in detergents and in the manufacture of phosphate fertilizers. Sulfur is a vital element for all forms of life. It is a component of two amino acids, cysteine and methionine.
Elemental sulfur is used in black gunpowder, matches, and fireworks; in the vulcanization of rubber; as a fungicide, insecticide, and fumigant; in the manufacture of phosphate fertilizers; and in the treatment of certain skin diseases. ... The most important sulfur compound is sulfuric acid.
Health effects of sulphur. All living things need sulphur. It is especially important for humans because it is part of the amino acid methionine, which is an absolute dietary requirement for us. ... Elemental sulphur is not toxic, but many simple sulphur derivates are, such as sulphur dioxide (SO2) and hydrogen sulfide.
Sulfur (S) is a bright yellow non-metallic element with an atomic number of sixteen. It is a naturally occurring element, but can also be extracted from common minerals. Interesting Sulfur Facts: Sulfur has been in use since ancient times and is mentioned in the Bible and the Torah.
Sulfur is low in toxicity to people. However, ingesting too much sulfur may cause a burning sensation or diarrhea. ... If animals eat too much sulfur, it may be toxic and can be fatal. Signs of poisoning in animals include problems to the stomach and intestines, effects on the lungs, and neurologic disorders.
The basics about the health benefits of sulfur: Sulfur-containing nutrients have tremendous health benefits, from maintaining healthy joints to boosting the immune system. Sulfur is found in every cell of the body. Many amino acids (protein building blocks), vitamins, and mineral compounds also contain sulfur.Dec 1, 2009
Arugula Carageenan Coconut milk, juice, oil Cruciferous veggies, including: bok choy, broccoli, cabbage, cauliflower, horseradish, kale, kohlrabi, mustard leaves, radish, turnips, watercress Dairy (except butter) Dried fruits Eggs Garlic Legumes and dried beans Lime/lemon juice in bottle Meat and fish Nuts Onions ( ...Nov 17, 2017
Eggs are not only a rich source of protein, they're high in sulfur, with the white, or albumen, containing the majority. Each egg yolk contains 0.016 milligram of sulfur, and the white contains 0.195 milligram, according to B. Srilakshmi, author of "Food Science."
Sulfur is an important part of several amino acids (the building blocks of protein), especially methionine and cysteine. It helps the body resist bacteria, cleanses the blood, and protects the protoplasm of cells. Key functions of sulfur include: Important in enzyme reactions and protein synthesis.




