Are alkaline solutions acidic or basic?
Best Answers
Because the base "soaks up" hydrogen ions, the result is a solution with more hydroxide ions than hydrogen ions. This kind of solution is alkaline. Acidity and alkalinity are measured with a logarithmic scale called pH. read more
Source: sciencebuddies.org
The "lost" hydrogen ions join up with water molecules to form hydronium ions (H3O+). For simplicity, hydronium ions are referred to as hydrogen ions H+. In pure water, there are an equal number of hydrogen ions and hydroxide ions. The solution is neither acidic or basic. read more
Source: sciencebuddies.org
A common task in chemistry labs is to identify whether a given solution is acidic, neutral or basic, which are determined by a solution's pH level. read more
Source: sciencing.com
Encyclopedia Research
Wikipedia:
Related Questions
Related Types
Image Answers
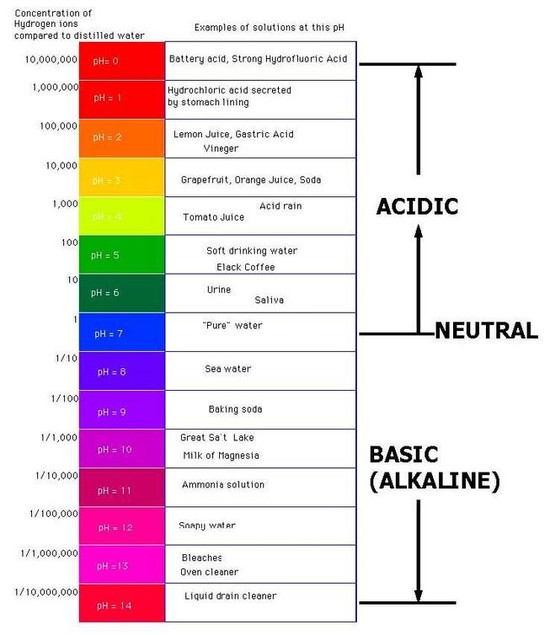
Source: sites.google.com
Further Research
Acids, Bases, & the pH Scale
www.sciencebuddies.org
Alkaline Vs. Basic
sciencing.com
Department of Chemistry
chemistry.elmhurst.edu
What is an Alkaline Solution?
www.livestrong.com



