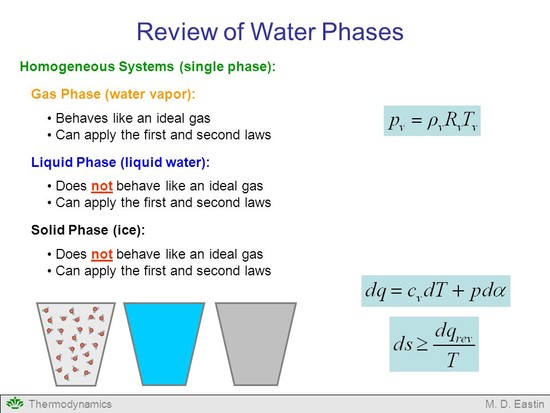
Can the ideal gas equation be applied to liquids like water?
Best Answers
No. The ideal gas theory depends upon a set of assumptions, notably that the molecules have zero volume and don't interact except during collisions. In a liquid, the molecules occupy nearly all the space and there are considerable forces between them. In fact liquids are essentially incompressible. read more
Truth be told, the ideal gas equation cannot be applied even to real gases perfectly. You need to assume many things before the ideal gas equation is applied to real gases. Liquids cannot be compressed as easily as gases can. Essentially, the compression amount is so low that it can be neglected. This is actually the principle behind hydraulics. read more
The ideal gas law is an equation of state, and some equations of state apply for liquids as well as gases. Besides, strictly speaking, "ideal gas law" wouldn't even suggest that the law could be used for vapors – but it works well for vapors when $P_r<<1$. And a saturated liquid is just a liquid at a temperature where it can boil. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers