How does acid rain increase the acidity of soil?
Best Answers
Soil contains a number of buffers: substances that can exist in weakly acidic or basic forms and are present as a mixture of both. Examples would but humic acid (+humates) and the various ionization states of phosphate. read more
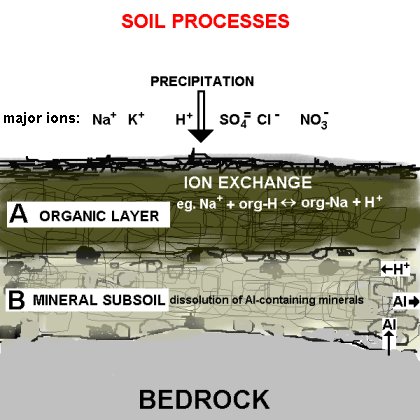
The corrosive effect of acid rain on soil is seen famously on the bald peaks of the Appalachian Mountains. The soil of these peaks is soaked from acidic fog, as are many high-elevation forests. While rain is always somewhat acidic due to oxidation, air polluted with nitrogen oxides and sulphur dioxide increase the pH of the rain to a harmful level. read more
Different types of bedrock contain variable amounts of alkaline chemicals. Regions with bedrock containing less alkali have a lower capacity for reducing acidity, and thus are more sensitive to acid deposition. Effects of soil on vegetation. When acid rain falls, it can affect forests as well as lakes and rivers. read more
Rain leaches alkaline elements including calcium, magnesium and potassium from the soil into runoff water, leaving acidic elements like hydrogen, aluminum and manganese to replace the bases. This means that areas with high annual rainfall amounts, such as parts of New England, generally have more acidic soil than the arid deserts of Arizona. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers