How does ammonium sulfate react with barium nitrate?
Best Answers
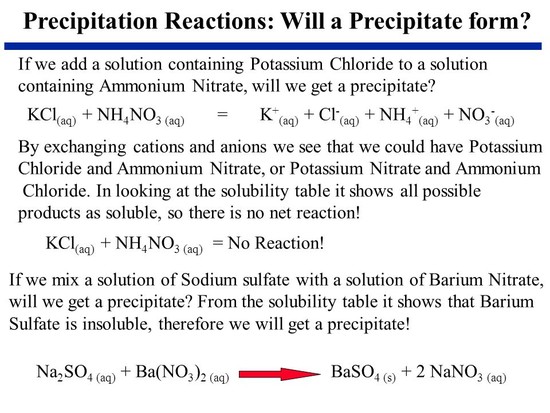
When aqueous solutions of these two salts are mixed, a heavy, white precipitate of barium sulfate is formed by double decomposition. Ba(NO3)2(aq) + (NH4)2SO4(aq) = BaSO4(s) + 2NH4NO3(aq) Ammonium nitrate, the other product, remains in the solution. read more
That is a double displacement reaction, where ammonium nitrate is produced with barium sulfate as a precipitate. The barium sulfate is going to be solid, which doesn't dissolve in water and odorless. Hope this helps. read more
If the teacher said: barium nitrate is added to ammonium sulphate, what happens - I would think very little if the powders alone were mixed. Water is required to form dissociated ions, which then react. read more
Ammonium sulfate reacts with barium nitrate to form ammonium nitrate and barium sulfate. (nh4)2so4 + ba(no3)2 ==> 2nh4no3 + baso4 It is a double replacement reaction. read more
Encyclopedia Research
Related Questions
Related Facts
Related Question Categories
Image Answers