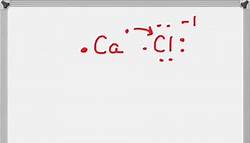How does the bond of calcium and chlorine form?
Best Answers
Calcium and chlorine mainly form ionic bonds. Ionic bonds arise from the electrostatic forces of attraction between the Ca2+ ion and two Cl- ions, hence forming CaCl2 salt. read more
Calcium chloride, or CaCl2, is composed of two Cl- anions ionically bound to a central calcium atom. Calcium has a 2+ charge, and the two chlorine ions each have a 1- charge. Forming ionic bonds with calcium, the chlorine ions achieve full valence electron shells and satisfy the octet rule. read more
calcium will bond with the other elements in the alkaline earth metal family. that would include magnesium, barium. radium, strontium, and beryllium. it may also bond with other elements like chlorine and sodium, but I'm not sure on that one. read more
Best Answer: The electronegative difference between Cl and Ca is 2.0. So, the bond is an ionic bond. The formula of the compound calcium chloride is CaCl2. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers