How many atoms are in glucose?
Best Answers
Molecules of Glucose= (5.23 g Glucose) x (1 mol Glucose/180.0 Glucose) x (6.02 x 10^23 molecules of Glucose/1 mol Glucose) = 1.75 x 10^22 molecules of Glucose. read more
So for number one you have 3.01 x 10^23 atoms. Divide that by a mol (6.02 x 10^23) and you have your answer. (.5 moles btw, so you can make sure you did it correctly) For number two: An atomic mass of any element is just saying how much one mole of the element weighs. So 6.02 x 10^23 atoms of cobalt weighs 58.93 grams. read more
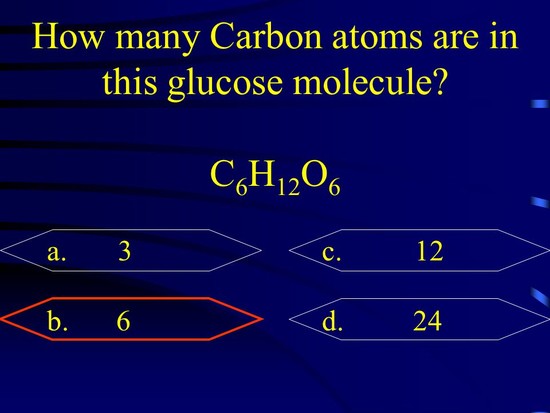
Calculation of molar mass of glucose (C 6 H 12 O 6) The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. From the molecular formula, C 6 H 12 O 6, one can find there are 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers