How was the Thomson model discovered?
Best Answers
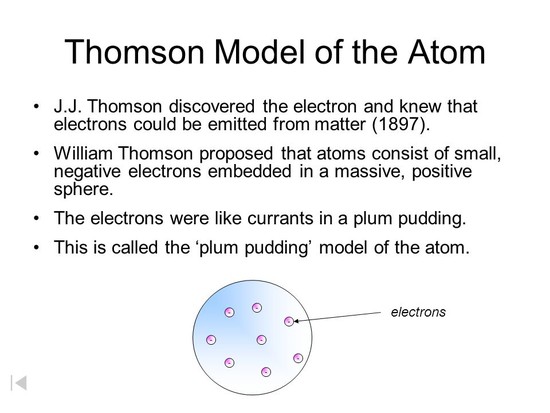
The British physicist Joseph John (J. J.) Thomson (1856–1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time. read more
Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom. read more
The plum pudding model is one of several scientific models of the atom. First proposed by J. J. Thomson in 1904 soon after the discovery of the electron, but before the discovery of the atomic nucleus, the model represented an attempt to consolidate the known properties of atoms at the time: 1) electrons are negatively-charged particles and 2) atoms are neutrally-charged. read more
The discovery of the electron disproved the part of Dalton's atomic theory that assumed atoms were indivisible. In order to account for the existence of the electrons, an entirely new atomic model was needed. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers