Is smelting of Iron ore endothermic or exothermic?
Best Answers
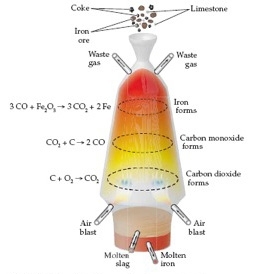
Smelting of iron ore is an exothermic process. Fe2O3 + 3CO = 2Fe + 3CO2 + 22.7 kJ. Delta-H (change in enthalpy) for the reaction is -22.7 kJ. Smelting is a redox reaction as it involves the reduction of a compound of a metal, usually an oxide, to free metal. But all redox reactions are not exothermic though most are. read more
Neither endothermic nor exothermic. These two terms apply to chemical reactions, wether they require or produce energy (heat). Melting iron isn't a chemical but a physical reaction, a change of phase, solid to liquid. read more
Source: answers.com
Encyclopedia Research
Wikipedia:
Related Questions
Related Facts
Related Types
Image Answers
Source: camelclimatechange.org
Further Research
endothermic reactions used in the gold mining industry
www.irsil.org
Smelt reduction for iron and steel sector
www.climatetechwiki.org
The Extraction of Iron
chem.libretexts.org