What happens when carbon monoxide is heated with zinc oxide?
Best Answers
Zinc oxide ([math]ZnO[/math]), an important type of semiconductor [1], also has a high chemical sensitivity to adsorbed gases making it useful as a gas sensor. read more
Nothing in particular. Both compounds are oxides, so neither has anything that could be exchanged with the other. read more
Zinc metal is extracted from zinc oxide by removing the oxygen atoms from zinc this is one of the method to get metals from metal oxide. Carbon can remove the oxygen atoms from metals this method is called reduction by “roasting” metal oxide with coke and metal is left and carbon dioxide is produced. read more
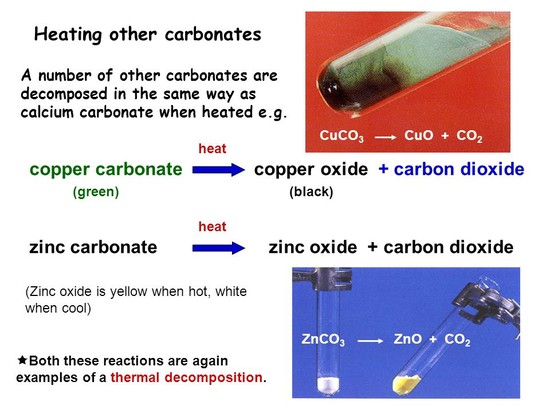
The reason for the yellow colour is that a minute amount of oxyegen evaporates from the lattice (70 ppm) the small number of zinc atoms produce lattice defects that give rise to the colour. Doping zinc oxide with minute traces of zinc will give a range of colours, yellow, green brown and red. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers