What happens when chloroform (CHCl3) reacts with Aqueous KOH?
Best Answers
When chloroform reacts with aq. KOH, the chlorines on the carbon atom are successively replaced by -OH groups from KOH via. nucleophilic reaction (SN2). In theory, first it forms CHCl2(OH), then CHCl(OH2), and then CH(OH)3, while eliminating KCl with each step. read more
When acetone reacts with chloroform, chloretone is formed as a product of the reaction. [math]CH3-CO-CH3 + CHCl3 -> (CH3)2-COH(CCl3)[/math] The anions of the reactants CH3CO- and CCl3- form bonds with cations H+ and CH3+ respectively, as a result forming chloretone. read more
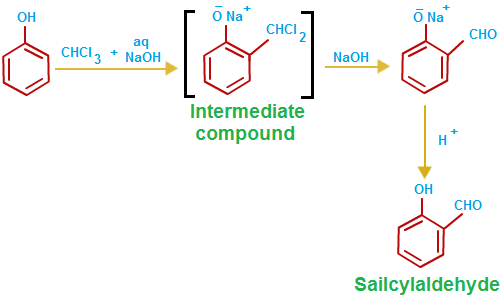
Chloroform reacts with aqueous sodium hydroxide (preferably in the presence of a phase transfer catalyst) to produce dichlorocarbene. This is used to effect ortho-formylation of activated aromatic rings such as phenols, producing aryl aldehydes in a reaction known as the Reimer-Tiemann reaction. read more
Encyclopedia Research
Related Questions
Related Facts
Related Question Categories
Image Answers