What happens when metals react with non metals?
Best Answers
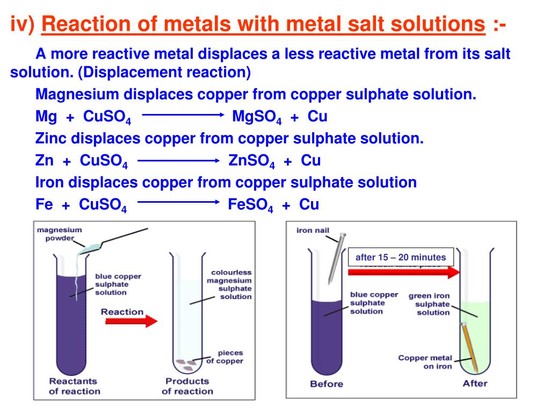
When a metal reacts with a non-metal it forms an ionic bond. Explanation: As we know metals have a tendency to lose electrons and form positive ions(cations).Which makes them electropositive. read more
Metal and non-metals oxides. Reaction with oxygen. Metals react with oxygen in the air to produce metal oxides, like magnesium oxide. Non-metals react with oxygen in the air to produce non-metal oxides. Here are two examples for the non-metals carbon and sulfur. Carbon burns in air to form carbon. read more
Reactions of other non-metals with bases are actually quite complex. Non-metal oxides are usually acidic in nature and react with bases in the same way as acids (salt and water). CO2 + 2NaOH —————-> Na2CO3 + H2O. There are more complex base-non-metal reactions such as chlorine with calcium hydroxide and chlorine with potassium hydroxide. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers