What is dalton's atomic theory?
Best Answers
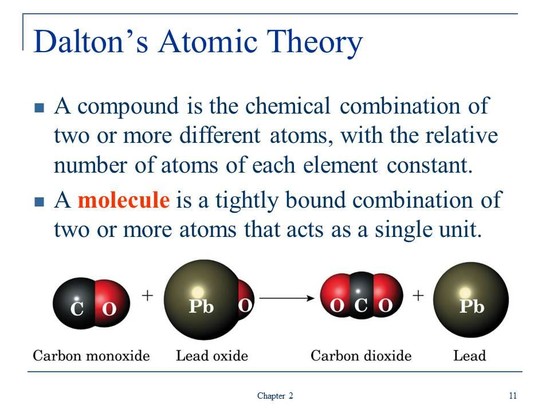
Dalton's Atomic Theory. 1) All matter is made of atoms. Atoms are indivisible and indestructible. 2) All atoms of a given element are identical in mass and properties. 3) Compounds are formed by a combination of two or more different kinds of atoms. read more
Modern atomic theory is, of course, a little more involved than Dalton's theory but the essence of Dalton's theory remains valid. Today we know that atoms can be destroyed via nuclear reactions but not by chemical reactions. read more
Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton based his theory on the law of conservation of mass and the law of constant composition. read more
Dalton's Atomic Theory. 1. Each chemical element is composed of extremely small particles that are indivisible and cannot be seen by the naked eye, called atoms. Atoms can neither be created nor destroyed. Pictured below is a helium atom. The purple and red dots represent the neutrons and protons in the nucleus. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers