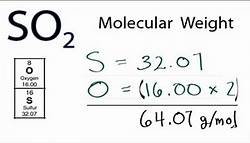What is the atomic mass of sulfur?
Best Answers
The highest value of the atomic weight of sulfur reported in the literature is from sulfate in reduced-sediment pore water undergoing sulfate reduction that had δ 34 S = +135 ‰ [x(34 S) = 0.0473 and A r (S) = 32.075]. read more
#color(blue)("avg. atomic mass" = sum_i("isotope"_i xx "abundance"_i))# In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance, which is simply the percent abundance divided by #100#. read more
Source: socratic.org
Encyclopedia Research
Wikipedia:
Related Questions
Related Facts
Related Types
Image Answers

Source: slideshare.net
Further Research
Atomic Weight of Sulfur
www.ciaaw.org
Molecular weight of Sulfur
www.convertunits.com