What is the atomic number for carbon?
Best Answers
Isotopes are atoms with the same number of protons but different numbers of neutrons. Carbon-12, carbon-13 and carbon-14 are three isotopes of carbon. They all have 6 protons but 6, 7 and 8 neutrons respectively. read more
Atomic number, atomic mass, and relative atomic mass Atoms of each element contain a characteristic number of protons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); the number of protons in an atom is called the atomic number. read more
The Periodic Table list the atomic numbers of the 100 or so known elements. It will certainly tell you the atomic number of carbon. read more
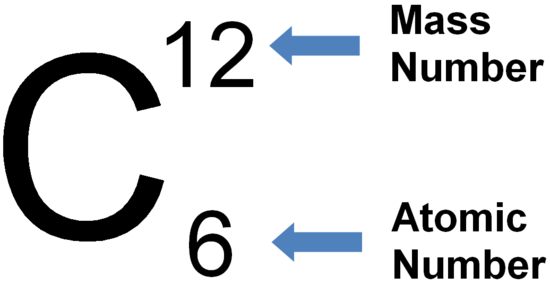
Carbon's atomic number is 6, and its atomic mass is 12.0107 atomic mass units. The atomic number defines the number of protons located in the atom's nucleus, so carbon has six protons. Each element has its own atomic number. read more
Atomic Number of Carbon is 6. Atomic Number is the Number of Protons in the nucleus of an atom. It has 6 Protons in the nucleus & 6 Elections orbitting around the nucleus. Typically the number of neutrons in Carbon is 6 (Carbon-12). But there are isotopes of Carbon having 7 (Carbon-13) & 8 (Carbon-13 ). read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers