What is the bohr model of the atom?
Best Answers
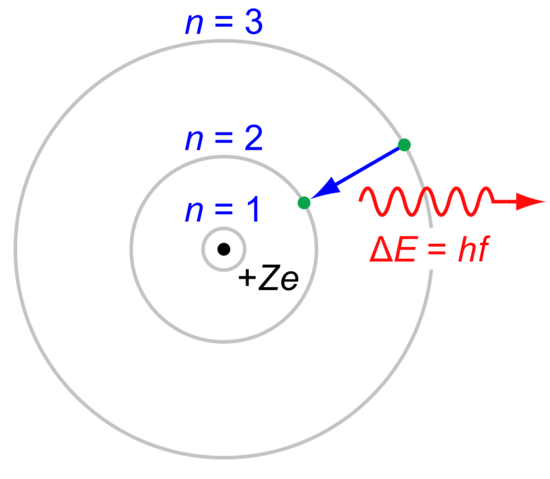
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively-charged electron orbits a small positively-charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. read more
Niels Bohr proposed the Bohr Model of the Atom in 1915. Because the Bohr Model is a modification of the earlier Rutherford Model, some people call Bohr's Model the Rutherford-Bohr Model. The modern model of the atom is based on quantum mechanics. read more
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers