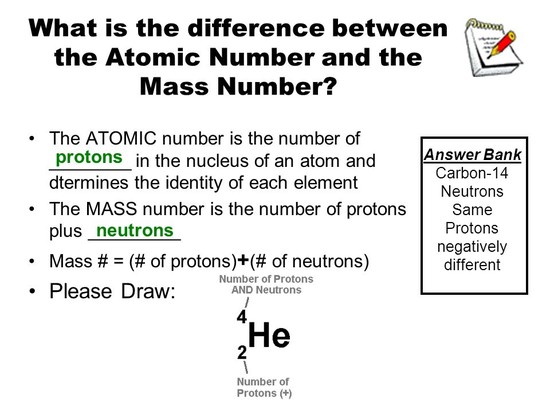
What is the difference between atomic mass and atomic weight?
Best Answers
The mass of an atom expressed in atomic mass units. The atomic weight of an element having more than one principal isotope is calculated both from the atomic masses of the isotopes and from the relative abundance of each isotope in nature. ... Compare atomic mass. See also mass number. read more
The atomic mass is expressed in units of atomic mass unit, or amu, and consists primarily of the protons and neutrons in the atom's nucleus, making it closely match the mass number of an atom. When an element has two or more isotopes, the atomic weight is expressed as an interval with an upper and lower number. read more
“An atomic weight (relative atomic mass) of an element from a specified source is the ratio of the average mass per atom of the element to 1/12 of the mass of an atom of 12C.” The weights given in the periodic table are calculated like this, and they are given in relative atomic mass. read more
The difference between atomic weight and atomic mass became known when F.W. Aston, the inventor of the mass spectrometer (1927) used his new device to study neon. At that time, the atomic weight of neon was believed to be 20.2 amu, yet Aston observed two peaks in the mass spectrum of neon, at relative masses 20.0 amu an 22.0 amu. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers