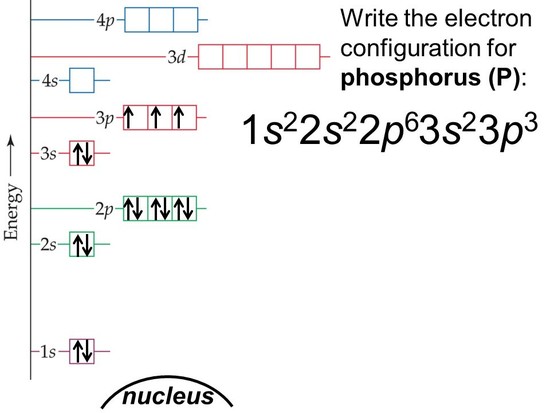
What is the electron configuration of phosphorus?
Best Answers
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Phosphorus (P). read more
Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Video: Phosphorus Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. read more
The valence electron configuration for phosphorus is #s^2 p^3#. Phosphorus has an electron configuration of #1s^2 2s^2 2p^6, 3s^2 3p^3#. Phosphorus is found in group 15, the other non-metals on the periodic table. Phosphorus is in the 3rd energy level, (3rd row) and 3rd column of the 'p' block #3p^3#. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers