What is the mechanism of hydration of alkenes?
Best Answers
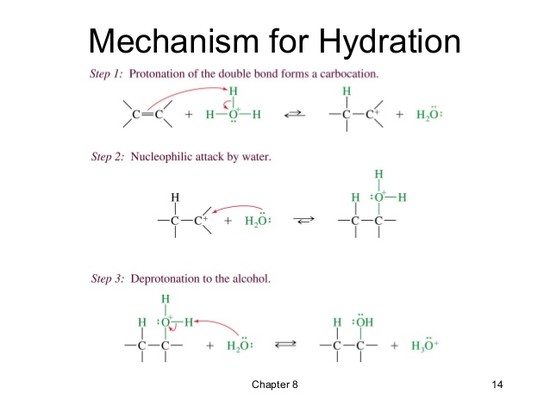
hydration of alkenes. Reaction type: Electrophilic Addition. Summary. When treated with aq. acid, most commonly H2SO4, alkenes form alcohols. Regioselectivity ... MECHANISM FOR REACTION OF ALKENES WITH H3O+. Step 1: An acid / base reaction. Protonation of the alkene to generate the more stable carbocation. read more
Electrophilic hydration is the act of adding electrophilic hydrogen from a non-nucleophilic strong acid (a reusable catalyst, examples of which include sulfuric and phosphoric acid) and applying appropriate temperatures to break the alkene's double bond. read more
Hydration of an alkene. The carbon-carbon double bond is converted to a single bond with a hydroxyl substituent. read more
Hydration of alkene is one of the important methods of preparation of alcohols. In this method alkenes are treated with conc.H₂SO₄ to form alkyl hydrogen sulphates which on boiling with water,alcohols are obtained alongwith H₂SO₄. Addition of acid on unsymmetrical alkenes takes place according to Markownikoff’s rule. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers