When ester reacts with sodium hydroxide what is formed?
Best Answers
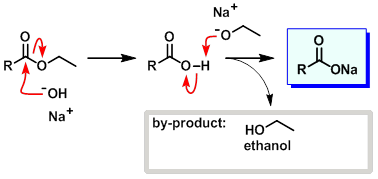
Esters have a common formula of ROOR' (R and R' are alkyl radicle). An ester for example ethyl ethanoate ( CH3COOC2H5) when reacts with sodium hydroxide( NaOH) forms sodium ethanoate (CH3COONa) as the main product and ethanol (C2H5OH). read more
An ester for example ethyl ethanoate ( CH3COOC2H5) when reacts with sodium hydroxide( NaOH) forms sodium ethanoate (CH3COONa) as the main product and ethanol (C2H5OH) This reaction is know as saponification reaction, alkaline hydrolysis or ester hydrolysis. read more
If the large esters present in animal or vegetable fats and oils are heated with concentrated sodium hydroxide solution exactly the same reaction happens as with the simple esters. A salt of a carboxylic acid is formed - in this case, the sodium salt of a big acid such as octadecanoic acid (stearic acid). read more
Encyclopedia Research
Related Questions
Related Facts
Related Question Categories
Image Answers