Which element is bigger, an atom of fluorine or of carbon?
Best Answers
It depends what you mean by bigger. As you go from left to right along a row in the periodic table, the atomic radius decreases as the additional electrons go into the outermost shell and are attracted by the increased positive charge in the nucleus. The covalent atomic radius of carbon is then 70 pm and of fluorine is 50 pm. read more
In terms of atomic radius, carbon is bigger than fluorine. Fluorine has a larger nucleus charge. Fluorine's electrons experience more pull. Thus fluorine is smaller. In terms of mass, a fluorine atom has a larger mass than a carbon atom. —Yuhan Zhang (Proud A-level Chemistry student. read more
Encyclopedia Research
Wikipedia:
Related Questions
Related Facts
Related Types
Image Answers
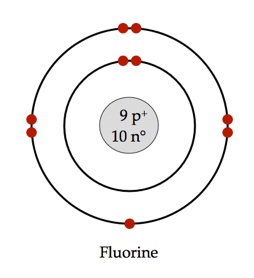
Source: pinterest.com
Further Research
Elements and Atoms
biology-pages.info
Facts, Discovery, Atomic Structure & Uses
www.livescience.com
