Why do atoms have no electric charge?
Best Answers
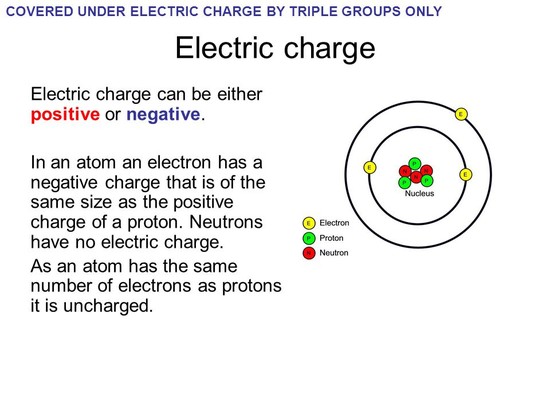
Why do atoms have no electric even though most of their particles have charges? Atoms have no electric charge because the protons and electrons "cancel out" each others charges. Neutrons have no charge. ... The atomic number is the number of protons in the atom's nucleus. read more
Neutrons are neutral, but protons and electrons are electrically charged. Protons have a relative charge of +1, while electrons have a relative charge of -1. The number of protons in an atom is called its atomic number. In the periodic table atoms are arranged in atomic number order. read more
Atoms have equal number of positively charged particles and negatively charged particles and some particles having no charge. Since numbers of positively charged particles and negatively charged particles are equal the NET charge as whole is zero. read more
Atoms have no electric charge because the protons and electrons "cancel out" each others charges. Neutrons have no charge. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Image Answers