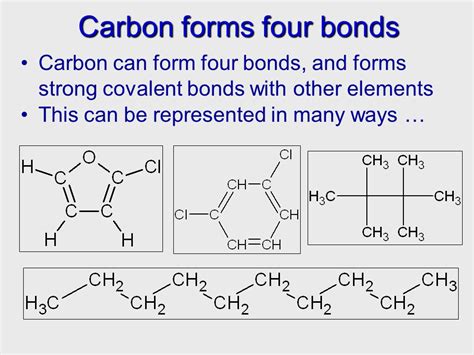
Why does carbon form covalent bonds?
Best Answers
As carbon is a non-metal, if it bonds with another non-metal then it will form covalent bonds, where the two non-metals share the electrons between themselves. Also, as carbon needs 4 extra electrons, it will form 4 of these covalent bonds to make itself stable. read more
Carbon atom has been no tendency to lose its four valence electrons or gain four more electrons from other atoms. Therefore, carbon atom completes its octet only by sharing its valence electrons with other atoms. As a result, therefore carbon always forms only covalent bonds with other atoms. read more
Covalent Bonds-Covalent bonding is a type of chemical bonding between 2 non metallic atoms that's characterised by the sharing of pairs of electrons between atoms and different covalent bonds. A covalent bond is formed between 2 non-metals that have comparable electronegativities. read more
Related Questions
Related Facts
Related Types
Image Answers