Why halogenation is not performed with iodine and flourine?
Best Answers
The halogenation with fluorine is may lead to explosion as fluorine is highly reactive element. And when we use iodine, the by product we get after halogenation is HI(Hydrogen Iodide) which is highly reactive and gets reduced. read more
The halogenation with fluorine is may lead to explosion as fluorine is highly reactive element. And when we use iodine, the by product we get after halogenation is HI(Hydrogen Iodide) which is highly reactive and gets reduced. So, the reaction becomes reversible. read more
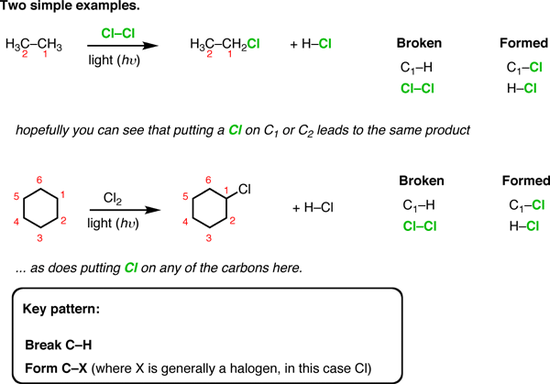
Radical Halogenation of Methane. The reactions of fluorine, chlorine, bromine, and iodine with methane are quite differently vigorous. Fluorine is the most reactive. If no precautions are taken, a mixture of fluorine and methane explodes. The reaction between methane and chlorine is easily controllable, while bromine is even less reactive than chlorine. read more
Encyclopedia Research
Related Questions
Related Facts
Related Question Categories
Image Answers