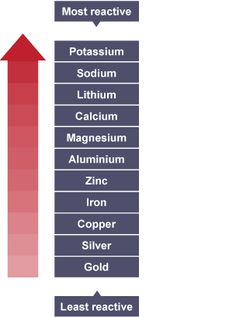
Why is francium and lithium not in the reactivity series?
Best Answers
They are, on some lists. For example, francium is second in the table, and lithium is sixth on the table in this article: Reactivity series - Wikipedia. The more commonly used tables are shortened, however, to make them easier to memorize and teac... read more
It's not correct that there elements are unstable - cesium and rubidium are stable (one of two natural isotopes of rubidium Rb-87 is indeed radioactive but only slightly - the other one Rb-85 is completely stable). read more
Francium has a $\ce{[Rn] 7s^1}$ electron configuration. It is a relatively heavy element with atomic number $Z=87$. In such heavy elements, relativistic effects become significant and impact the reactivity and other physical characteristics of the element. read more
Encyclopedia Research
Related Questions
Related Facts
Related Types
Related Question Categories
Image Answers