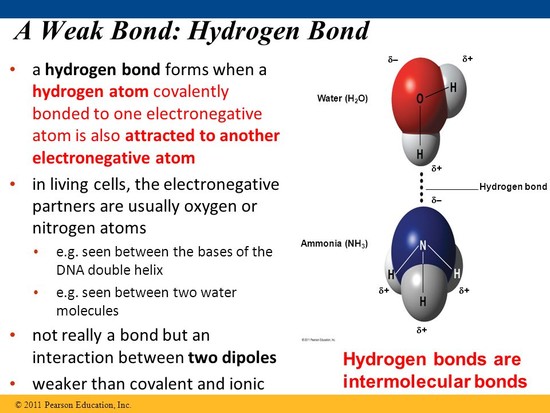
Why is hydrogen bond a weaker bond than covalent bond?
Best Answers
As has already been said, a hydrogen bond is a type of intermolecular bond that occurs when a hydrogen, bonded to a very electronegative atom (F, O or N) interacts with a hydrogen on another molecule. read more
Because hydrogen bonds involve no formal electron exchange, the interaction is weaker than in covalent bonds, where unless the bond is highly polarised, or there is a high enough energy provided, the bond will not easily dissociate. read more
Best Answer: Covalent bonding is when two atoms share electrons and the attraction holds them together, hydrogen bonding is much weaker because it is caused by the "partial polar" attraction of the hydrogen atom (like in a water molecule for example). read more
Related Questions
Related Types
Image Answers