Why is ionization enthalpy of indium less than gallium?
Best Answers
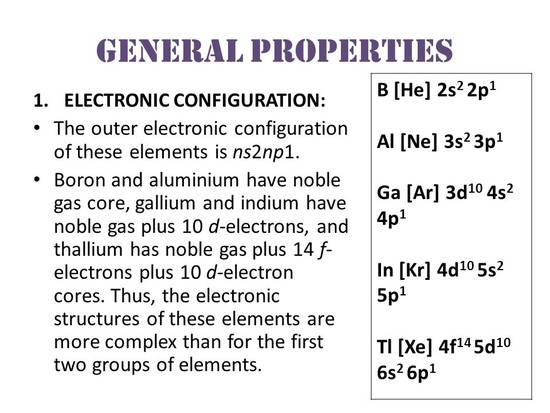
I think you are talking about first ionization energy. Lets talk only about the outermost electron & its I.E . Gallium- Electron Configuration- [Ar] [math]3d^{10} 4s^{2} 4p^{1}[/math] 1st ionization energy- 578.8 kJ/mol Indium- Electron configurat... read more
Z(effective) is very less for the outer most electron since since shielding constant is very high (to calculate see Slater's rule), so attraction with the nucleus is less for the outermost electron of “In” compared to “Ga” so the Ionization energy of Gallium is higher then “In” . read more
Indium and Aluminium come in the same group. And as we know that the ionization energy decreases down the group and Indium comes below Al, therefore, the ionization energy of Indium is lower than Al. Ionization energy is the energy used to remove an electron from the outermost shell of the atom. read more
The ionization enthalpy grdually decreses down the group in normal case. But in 13th group when we compare the ionisation enthalpy of Al and Ga, Ga has IE more than Al due to the inability of d and f orbitals so that screening effect is less and more nuclear charge on the valance shell electrons. read more
Related Questions
Related Facts
Related Types
Image Answers