Types of Radioactive Decay

Actinium is a rare element that is present in uranium ores in tiny amounts, but it is usually cheaper and easier to create actinium when it is needed by bombarding radium with neutrons in a nuclear reactor.

Radioactive decay is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons. Radioactive decay is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons.

Astatine is a radioactive chemical element with symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. A sample of the pure element has never been assembled, because any macroscopic specimen would be immediately vaporized by the heat of its own radioactivity.

In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta ray (fast energetic electron or positron) and a neutrino are emitted from an atomic nucleus. For example, beta decay of a neutron transforms it into a proton by the emission of an electron, or conversely a proton is converted into a neutron by the emission of a positron (positron emission), thus changing the nuclide type.
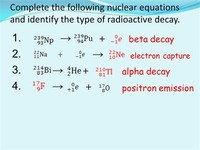
Electron capture is always an alternative decay mode for radioactive isotopes that do have sufficient energy to decay by positron emission. Electron capture is sometimes called inverse beta decay, though this term can also refer to the interaction of an electron antineutrino with a proton.

A radioactive decay, on the other hand, is a spontaneous event whereby the nucleus of an atom discharges a particle (alpha, beta, gamma, neutron, positron, etc), and changes its nuclear state. There are a few elements that undergo spontaneous fission, which is considered a radioactive decay and not a nuclear reaction.

It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22 minutes. It is the second-least electronegative element, behind only caesium, and is the second rarest naturally occurring element (after astatine).

Fission, Fusion, and Radioactive Decay Nuclear Energy Nuclear energy is the energy released in nuclear reactions. Two types of reactions that release huge amounts of energy are nuclear fission and nuclear fusion. Nuclear fission is the splitting of the nucleus of an atom into two smaller nuclei.
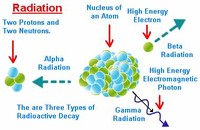
Radioactive decay is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons. Radioactive decay is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons.

Radioactive decay (also known as nuclear decay or radioactivity) is the process by which an unstable atomic nucleus loses energy (in terms of mass in its rest frame) by emitting radiation, such as an alpha particle, beta particle with neutrino or only a neutrino in the case of electron capture, gamma ray, or electron in the case of internal conversion.

Polonium is a chemical element with symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth.

Positron emission or beta plus decay (β + decay) is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (ν e). Positron emission is mediated by the weak force.

The primary decay products are neodymium and samarium isotopes (promethium-146 decays to both, the lighter isotopes generally to neodymium via positron decay and electron capture, and the heavier isotopes to samarium via beta decay). Promethium nuclear isomers may decay to other promethium isotopes and one isotope (145 Pm) has a very rare alpha decay mode to stable praseodymium-141.

Radium is a highly radioactive alkali earth metal, and, though some of the elements from this chemical group are critical for life (if you don't have them you die), this one is to be avoided if it is at all possible. Zero radium is a good amount to have in your body any time. All...

Radon is produced by the radioactive decay of radium-226, which is found in uranium ores, phosphate rock, shales, igneous and metamorphic rocks such as granite, gneiss, and schist, and to a lesser degree, in common rocks such as limestone.

The primary decay mode for isotopes lighter than technetium-98 (98 Tc) is electron capture, producing molybdenum (Z = 42). For technetium-98 and heavier isotopes, the primary mode is beta emission (the emission of an electron or positron), producing ruthenium (Z = 44), with the exception that technetium-100 can decay both by beta emission and electron capture.

One type of natural transmutation observable in the present occurs when certain radioactive elements present in nature spontaneously decay by a process that causes transmutation, such as alpha or beta decay. An example is the natural decay of potassium-40 to argon-40, which forms most of the argon in the air.